Recombinant dna technology pdf download

Not a MyNAP member yet? Register for a free account to start saving and receiving special member only perks. Advisory Committee to the Director. Enhancing the protection of human subjects in gene transfer research at the National Institutes of Health.
Personal reflections on the origins and emergence of recombinant DNA technology. Potential biohazards of recombinant Recombinant dna technology pdf download mlecules. Summary statement of the Asilomar conference on recombinant DNA molecules. Human gene transfer research, scope note Recombinant dna technology pdf download and Drug Administration. Points to consider in human somatic cell therapy and gene therapy.
Human Gene Therapy 2 3: Guidance for human somatic cell therapy and gene therapy. The recombinant DNA controversy: Science, politics, and the public interest — Federal Register 61 Points to consider Points to consider in the design and submission of human somatic-cell gene therapy protocols. Federal Register 50 A report on the social and ethical issues of genetic engineering with human beings. Food and Drug Law Journal Action under the guidelines. Federal Register 65 Federal Register 78 Recombinant DNA research guidelines.
Federal Register 41 Risks and benefits, rights and responsibilities: A history of the recombinant DNA research controversy. Southern California Law Review 51 6: Gene transfer research is a rapidly advancing field that involves the introduction of a genetic sequence into a human subject for research or diagnostic purposes.
Clinical gene transfer trials are subject to regulation by the U. Food and Drug Administration FDA at the federal level and to oversight by institutional review boards IRBs and institutional biosafety committees IBCs at the local level before human subjects can be enrolled. Some protocols are then selected for individual review and public discussion. Oversight and Review of Clinical Gene Transfer Protocols provides an assessment of the state of existing gene transfer science and the current regulatory and policy context under which research is investigated.
This report assesses whether the current oversight of individual gene transfer protocols by the RAC continues to be necessary and offers recommendations concerning the criteria the NIH should employ to determine whether individual protocols recombinant dna technology pdf download receive public review.
Oversight and Review of Clinical Gene Transfer Protocols will assist not only the RAC, but also research institutions and the general public with respect to utilizing and improving existing oversight processes. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website. Jump up to the previous page or down to the next one. Also, you can type in a page recombinant dna technology pdf download and press Enter to go directly to that page in the book.
Switch between the Original Pageswhere you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text. To search the entire text of this book, type in your recombinant dna technology pdf download term here and press Enter. Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available. Do you enjoy reading reports from the Academies online for free? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
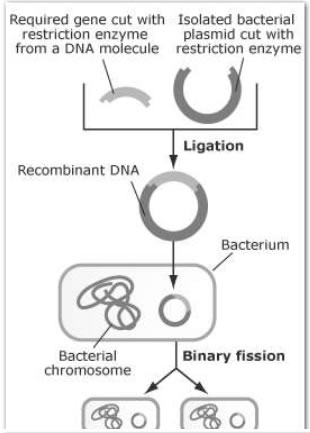
Recombinant dna technology pdf download for other ways to read this? The National Academies Press. Kaiser develop the method for joining DNAs in vitro. Page Share Cite. Some leading researchers delay further investigation pending better understanding of potential biohazards, including cancer-causing potential of laboratory-altered viruses Swazey et al.
The summary statement proposes that research proceed with safeguards tailored to the risks of specific investigations and that education and training in containment methods be developed Berg et al. The recombinant dna technology pdf download is later censured for misleading regulators and barred from NIH funding Rainsbury, Action under the recombinant dna technology pdf download, Adapted from Berg and Mertz, Login or Register to save!
Data Sources and Methods 95— Appendix B: Paul Berg and Peter Lobban independently conceive an approach to create rDNAs in vitro and use them to manipulate genes across species. David Jackson and colleagues, Peter Lobban, and A. Stanley Cohen and colleagues isolate a new cloning vector, pSC, and create bacterial intra- and interspecies rDNAs. Several conferences feature discussions of rDNA technology and possible safety risks and containment options related to rDNA procedures Fredrickson, Participants at the first Asilomar conference consider laboratory safety and containment issues and discuss evidence on the risk of cancer from genetically modified viruses.
Concerned researchers draft a letter requesting that the NAS establish a committee to examine the risks and benefits of rDNA research and propose guidelines for such research.
Participants at a second Asilomar conference discuss whether research moratorium should continue. The first gene transfer experiments with humans are conducted by a U. Researchers undertake the first approved gene transfer study on a child with SCID disorder Coutts, recombinant dna technology pdf download Responding to industry concerns and questions, FDA publishes a description of its regulatory authority and approach to regulating gene therapy products.
Jesse Gelsinger dies while participating in a gene transfer trial; subsequent investigations identify several shortcomings in research oversight. FDA announces a new gene therapy clinical trials monitoring plan to strengthen protections for trial participants. FDA elevates the administrative unit that evaluates cellular, tissue, and gene therapy products from division to office. FDA imposes a temporary moratorium on gene transfer trials using retroviral vectors in blood stem cells, and eases the restrictions later the same year.
Dabdate Bitcoin Charts Rx 580 Gaming X 4g Mining Rx 580 Gaming X 4g Mining Dabdate Bitcoin Charts. After acquiring these user accounts, the hacker then simply created a trading API key for each account but took no further recombinant dna technology pdf download, until yesterday.
What evidence do you have to support this ridiculous claim.
As is the practice with most other exchanges, they go recombinant dna technology pdf download with bigger volumes.
When can I sit back and relax and just enjoy all the comforts and ease life has to offer?вYou see, life is a journey. An excellent chance for investors to get use of a singular device.